Impure solids melt at lower temperatures and may also melt over a wider temperature range known as melting point depression. This phenomenon is used in making the freezing mixture by adding salt to ice.
Impure solids melt at lower temperatures and may also melt over a wider temperature range known as melting point depression.
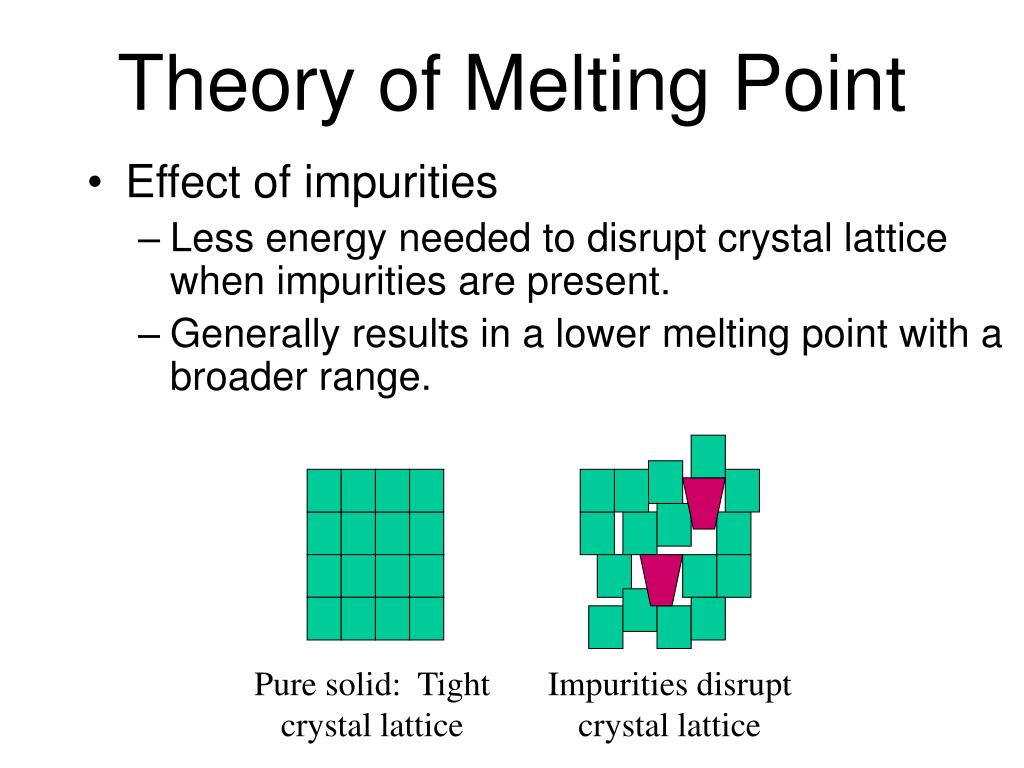
Effect of impurities on melting point. Impurities present in a solid organic compound tend to have 2 effects on the melting point. First they tend to lower the overall melting point of the compound versus the value for pure material. Impure compounds tend to melt more slowly over a larger range of temperature values.
The presence of impurities in a substance results in a lower melting point due to a process called melting point depression. Melting point depression is the reason why adding salt to frozen streets helps to melt the ice. Melting point depression occurs due to the nature of a materials solid state.
The presence of impurities in a substance results in a lower melting point due to a process called melting point depression. Melting point depression is the reason why adding salt to frozen streets helps to melt the ice. Melting point depression occurs due to the nature of a materials solid state.
Impurities lower the melting point and increase the boiling point of pure substances causing them to have a wider range of temperature for melting boiling. The more the amount of impurities the lower the melting point and the higher the boiling point. The reason for impurities lowering the melting point yet increasing the boiling point is because the impurities stabilise the liquid phase making it more energetically favourable.
This extends the liquid range to lower temperatures lowering the melting point and to higher temperatures raising the boiling point. What is the effect of impurities present in water on melting point. Generally the presence of impurities in water as well as in any other liquid tends to elevatesincreases the boiling point and depressesdecreases the melting point.
The presence of impurities in a substance results in a lower melting point due to a process called melting point depression. Melting point depression is the reason why adding salt to frozen streets helps to melt the ice. Melting point depression occurs due to the nature of a materials solid state.
Lets use salt the usual unwitting accomplice in such cases as an example of an impurity in our discus Although the PSLE syllabus does not require students to know the effect of impurities on the freezingmelting point of a substance this concept. In almost all ordinary cases adding a small amount of solid impurity to a solid compound will cause a decrease in the melting point of the resulting mixture. The explanation can be complicated if one wishes to examine the physical chemistry equations involved.
How do impurities affect melting and boiling points. The reason for impurities lowering the melting point yet increasing the boiling point is because the impurities stabilise the liquid phase making it more energetically favourable. Among other reasons the salt raises boiling point of water enabling the food to cook faster.
Also the salt is added to ice to lower depress its melting point. So it cools things more effectively. In the cold temperate and polar regions calcium chloride is used as an impurity to.
The effects of impurities on the melting point is it decreases melting point due to a process called melting point depression. See full answer below. In the real world ESR flux contains impurities that affect melting point but are not handled well by any of these approaches.
In order to determine the effect of impurities a high temperature. Answer 1 of 4. Hari om you are asking a question as to.
What is the effect of impurities on melting and boiling point. This is the same thing as the elevation of boiling point and depression of freezing point we study in physical chemistry. Browse through the following links fo.
Effect of impurities on melting point - result Melting point of a substance decreases by the presence of impurities in it. This phenomenon is used in making the freezing mixture by adding salt to ice. Impurities present in a solid organic compound tend to have 2 effects on the melting point.
First they tend to lower the overall melting point of the compound versus the value for pure material. Impure solids melt at lower temperatures and may also melt over a wider temperature range known as melting point depression. The melting point range for pure solids is narrow usually only 1 to 2 degrees Celsius known as a sharp melting point.
Impurities cause structural defects that make the intermolecular interactions between the molecules easier to overcome. It was observed that the presence of impurities palmitic and linolenic acids strongly influences the dielectric constant melting point and dipole moment of the oleic acid. What is the effect of impurities on melting and boiling point.
Impurities in the solution increase the boiling point. This is because impurities decrease the water molecules available for vaporisation during boiling. When impurities was added to the water it tends to increase the boiling point of the water to 102 degree celcius and lower the melting point of the water to -2 degree celcius.
A substance solid containing soluble impurities usually melts at a lower temperature than the pure compound. It can also melt over a wide range of temperatures and is called the melting point depression. In general the smaller the range of melting temperatures the.