
The forming of a covalent bond releases energy. A one minus Florian we find is in Group seven.
Part A Rb Express your answer as an ion.
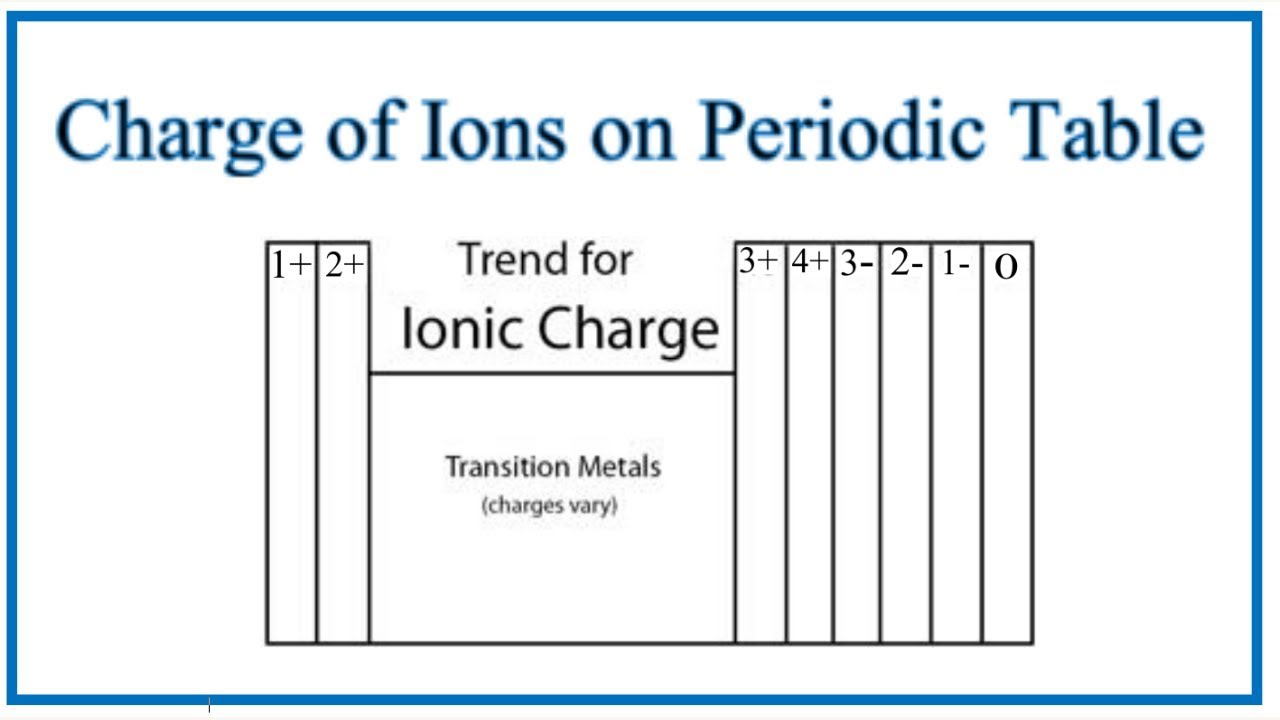
Predict the ion formed by each element. To predict the ion formed for each element. We simply need to identify in which group it is found fits in Group one A. It produces a one plus I on to a two plus three a three plus skip for a five a three minus 68 to minus seven.
A one minus Florian we find is in Group seven. A so one minus nitrogen is in Group five a so three minus my knees. Predict the charge of the ion formed by each element and write the electron configuration of the ion.
Next we have potassium thats a group one metal. So it would like to lose one electron and form a plus one iron in order to have a full valence shell. Next we have alum in ium and so this would like to lose three electrons to have a full valence shell.
When they gain electrons they are negatively charged and are named anions. You can predict the charge of an ion by looking at its group number on the periodic table. Groups IA IIA and IIIA all lose electrons and become positively charged.
How do you predict the charges of ions formed by main group elements. Get the detailed answer. Predict the ion formed by each element.
Part A Rb Express your answer as an ion. The octet rule explains how ions are formed. Learn about this rule and use it to predict an ions formation charge and formula and also understand the role of cations and anions in this process.
Sodium ions and calcium ions are examples ofA. When sodium atoms form sodium ions theya. An atom becomes an ion when its.
Predict the charge of the ion formed by each of the following elementsF. Predict the charge of the ion formed by each of the following elementsF. Predict the charge of the ion formed by each element and write the electron configuration of the ionPart A.
SeExpress your answer as an ionPart BWrite the electron configuration of the ion formed by SeExpress your answer in order of increasing orbital energy. For example the elctron configuration of. Example Exercise 121 Bond Predictions A metal ion and nonmetal ion are attracted in ionic bonds.
Two or more nonmetal atoms are attracted in covalent bonds. The forming of a covalent bond releases energy. Predict which element in each of the following pairs is more electronegative according to the general.
Feedback Resume Question 6 of 15 Attempt 1 Predict the charge on the stable ion formed by each element. 2 5 Incorrect T. 2 -2 Incorrect Assignment Score.
Feedback Resume Question 8 of 15 Attempt 1 Give the formula for an ionic compound formed from each pair of ions. Predict the monatomic ion formed from different elements based on the position in the periodic table. You can predict the charge of an ion by looking at its group number on the periodic table.
Groups IA IIA and IIIA all lose electrons and become positively charged. Groups VA VIA and VIIA all. Main group metals tend to lose electrons forming a cation with the same number of electrons as the nearest noble gas.
A main group nonmetal tends to gain electrons forming. The question is really just asking for the symbol of fluorine which is F- Another example would be if it asked Predict the ion formed by Na sodium The answer would simply be Na. Predict the charge of the ion formed by each of the following elements.
A Mg Express your answer as an ion. A chemical reaction does not occur for this question. B Cl Express your answer as an ion.
A chemical reaction does not occur for this question. C Br Express your answer as an ion. Solution for Predict the charge on the stable ion formed by each element.
Predict how many electrons will most likely be gained or lost by each element. Ga-It will lose 3 electrons-It will gain 4 electrons-It will gain 1 electron-It will gain 2 electron. Hence barium will acquire a 2 charge and forms ion.
On the other hand chlorine is a group 17 element and its electronic distribution is 2 8 7. Hence to attain stability it will gain one electron and forms ion. Thus we can conclude that charge on barium ion is 2 and charge on chlorine ion is -1.
2 lithium atoms 1 sulfur atom and 4 oxygen atoms. 1 aluminum atom and 3 bromine atoms. 2 sodium atoms 1 sulfur atom and 4 oxygen atoms.
1 magnesium atom 2 nitrogen atoms and 4 oxygen atoms. Write a chemical formula for each molecular model. The ions formed are negative because they have more electrons than protons.
The ions have the electronic structure of a noble gas group 0 element with a full outer shell. For elements in. Exercise 259 predict the charge of the ion formed by.
Exercise 259 Predict the charge of the ion formed by each of the following elements. Part A Express your answer as an ion. Answe r not display ed.
Part B Express your answer as an ion. Part C Express your answer as an ion. Answe r not display ed.