How many isomers of C5H12 are there. Pentane C 5 H 1 2 is an organic compound with five carbon atoms.
There are three isomers of a pentane including n-pentane isopentane and neopentane.
Three structural isomers of c5h12. This one examines the three different chain structural isomers of C5H12 including a loo. One in a series of videos looking at isomerism in organic chemistry. Pentane C5H12 is an organic compound with five carbon atoms.
Pentane has three structural isomers that are n-pentane Iso-pentane methyl butane and neopentane dimethylpropane. Structural isomers in C5H12. Three structural isomers have the formula C5H12.
Draw and name the isomers using IUPAC rules. By signing up youll get thousands of. An Isomer of a compound are other compounds that have the same formula with the original compound but have different arrangements.
Pentane as 2 other isomers. Answer 1 of 3. The molecular formula for pentane is C5H12.
It is an organic compound as well as a hydrocarbon. There are five atoms of carbon and twelve atoms of hydrogen present in one molecule of pentane. The molar mass of pentane is 72 grams per mole.
There are three isomers of a pentane including n-pentane isopentane and neopentane. Click hereto get an answer to your question How many isomers can C5H12 have. Join Login Class 11 Chemistry Organic Chemistry - Some Basic Principles and Techniques Isomerism.
Correct option is. C 5 H 1 2 can have 3 isomers. B Name the type of structural isomerism shown by the isomers of C 5 H 12 _____ 1 c 2-Hydroxypropanenitrile displays optical isomerism.
Draw three-dimensional representations of the two enantiomers of 2-hydroxypropanenitrile showing how the two structures are related to each other. Three structural isomers have the formula C5H12. Draw and name the isomers using IUPAC names.
5C atoms in main chain B. 4C atoms in main chain C. 3C atoms in main chain.
The molecular formula for pentane is C5H12. N-pentane 2-methylbutane and 2-ethylpropane are three structural isomers of pentane. Additionally how many stereoisomers are possible for chlorination of c5h12.
6 There are three constitutional isomers with molecular formula C5H12. Chlorination of one of these isomers yields only one product. Pentane C5H12 is an organic compound with five carbon atoms.
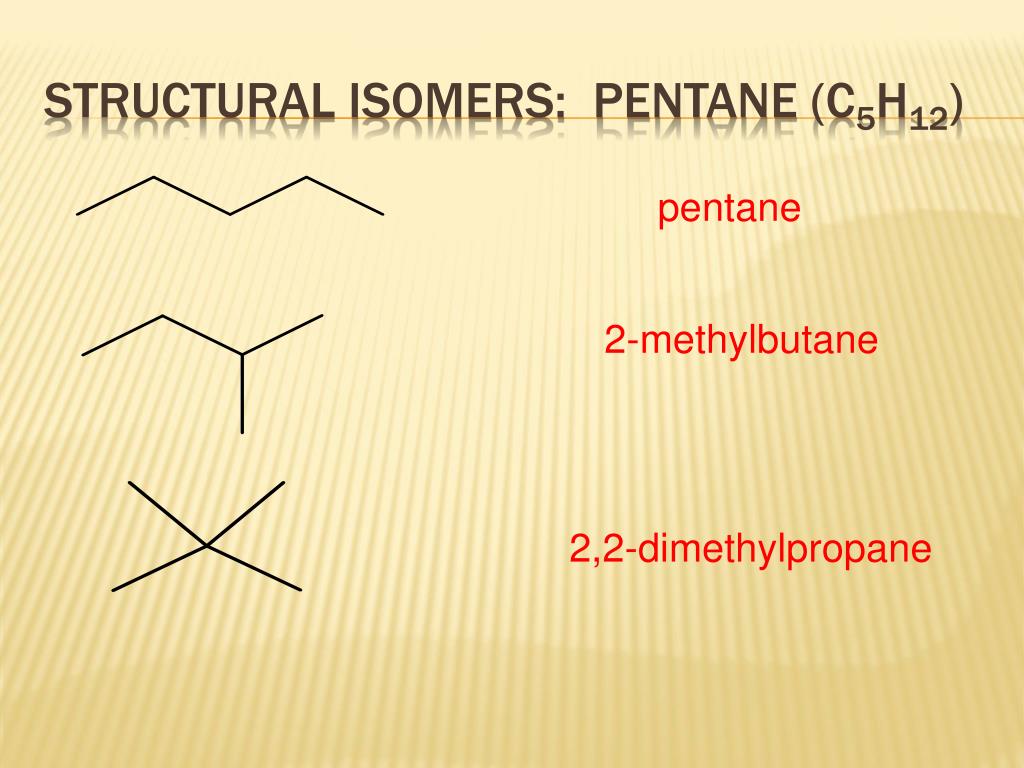
Pentane has three structural isomers that are n-pentane Iso-pentane methyl butane and neopentane dimethylpropane. Pentane C 5 H 12 has three structural isomers. Isomer 1 is n-pentane the straight chain normal structure for pentane.
Isomer 2 is 2-methylbutane a branched chain with a carbon atom joined onto three other carbon atoms. Isomer 3 is 22-dimethylpropane a branched chain with the central carbon atom joined onto four other carbon atoms. Note that for all the isomers.
Pentane C 5 H 12 is an organic compound with five carbon atoms. Pentane has three structural isomers that are n-pentane Iso-pentane methyl butane and neopentane dimethylpropane. Therefore three structural isomers can be drawn from pentane.
C5H12 has 3 isomers in total they are pentane 2-methylbutane and 22-dimethylpropane. This video shows a systematic way of drawing all three constitutional. The isomers of C5H12 are.
N - pentane 2 - methyl pentane 22 - dimethyl pentane. The structural formula of an organic compound shows the arrangement of atoms and groups in the compoundA particular molecular formula could have different arrangements of atoms in space. Three structures could have the formula C5H12 as shown in the image attached to this answer.
There are three different structural isomers for a hydrocarbon with the formula c5h12. They are pentane isopentane and neopentane. Atom we are back up to a three carbon chain and we just did that.
So there are three different skeletal or structural or constitutional isomers for our saturated formula of C5H12. Drawing all possible isomers of the five examples thus far has not proven particularly difficult. CH4 C2H6 C 3H8 C4H10 C5H12 1 isomer 1 isomer 1 isomer 2 isomers 3 isomers.
Chain isomers are molecules with the same molecular formula but different arrangements of the carbon skeleton. Pentane C 5 H 1 2 is an organic compound with five carbon atoms. Pentane has three structural chain isomers that are n-pentane Iso-pentane methyl butane and neopentane dimethylpropane.
Pentane C5H12 is an organic compound with five carbon atoms. Pentane has three structural isomers that are n-pentane Iso-pentane methyl butane and neopentane dimethylpropane. In C5H12there are total 3 constitutional isomers.
1 n-pentane pentane 2 iso-pentane 2-methylbutane 3 neopen. View the full answer. Previous question Next question.
How many isomers of C5H12 are there. 3 isomers C5H12 can have 3 isomers. What are the 5 structural isomers of C6H14.
The five isomers possible for hexane are n- hexane 2- methyl pentane 3- methyl pentane 2 3-dimethylbutane and 2 2- dimethylbutane. 2- methyl pentane is also called Isohexane. Three structural isomers have the formula C5H12C5H12.
Draw and name the isomers using IUPAC names. Draw the isomer with five carbon atoms in main chain. Lets say were asked to draw all the structural isomers that have the molecular formula c5h12 the word isomer means same parts and so were talking about the same number of atoms all of our structural isomers are going to have five carbons and 12 hydrogens our isomers are going to differ and how those atoms are connected to each other so they differ in terms of their structure and thats why we call them structural isomers you can also call them constitutional isomers.