
At a given temperature say T 1 the transformation starts after an incubation period t 2 at T 1. Isothermal transformation moves the curve toward the right at any temperature higher than about 482C 900F.
After 50 transformation locus of that time t 3 at T 1for different temperatures is called 50 transformation line.
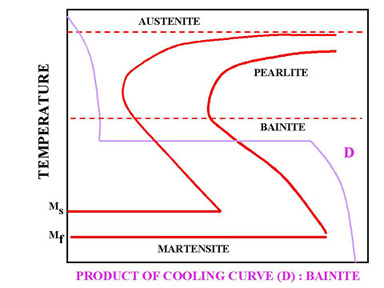
Time temperature transformation curve. The Time t Temperature t Transformation Curve TTT At slow cooling rates the trajectory can pass through the Pearlite and Bainite regions Pearlite is formed by slow cooling Trajectory passes through Ps above the nose of the TTT curve Bainite Produced by rapid cooling to a temperature above Ms Nose of cooling curve avoided. Time-Temperature-Transformation Curves TTT curves are a way of plotting transformation kinetics on a plot of temperature vs. A point on a curve tells the extent of transformation in a sample that is transformed isothermally at that temperature.
A TTT diagram usually shows curves that connect points of equal volume fraction transformed. T Time T Temperature T Transformation diagram is a plot of temperature versus the logarithm of time for a steel alloy of definite composition. It is used to determine when transformations begin and end for an isothermal constant temperature heat treatment of a previously austenitized alloy.
When austenite is cooled slowly to a temperature. Time-Temperature-Transformation TTT diagram or S-curve refers to only one steel of a particular composition at a time which applies to all carbon steels. This diagram is also called as C-curve isothermal decomposition of austenite diagram and Bains curve.
The time-temperature transformation curves correspond to the start and finish of transformations which extend into the range of temperatures where austenite transforms to pearlite. Above 550 C austenite transforms completely to pearlite. Below 550 C both pearlite and bainite are formed and below 450 C only bainite is formed.
TTT diagram of steel indicates the time-temperature and transformation curve. This means transformation is dependent upon time temperature and cooling mechanism. Difference between phase diagram and TTT diagram of steel.
For a clear understanding of this diagram it is better to understand the phase transformation of the Fe-Fe3C curve. The relationships between the extents of conversion at gelation and at vitrification and the isothermal cure temperature form the basis of a theoretical model of the timetemperaturetransformation TTT cure diagram in which the times to gelation and to vitrification during isothermal cure versus temperature are predicted. Time-Temperature-Transformation TTT Curves TTT diagram is a plot of temperature versus the logarithm of time for a steel alloy of definite composition.
TTT diagram indicates a specific transformation starts and ends and it also shows what percentage of transformation of austenite at a particular temperature is achieved. At a given temperature say T 1 the transformation starts after an incubation period t 2 at T 1. Locus of t 2 for different for different temperature is called transformation start line.
After 50 transformation locus of that time t 3 at T 1for different temperatures is called 50 transformation line. While transformation completes that time t 4 at T 1. Isothermal transformation moves the curve toward the right at any temperature higher than about 482C 900F.
That is above what has been called the nose or knee of the beginning curve. This retardation is reflected in the greater hardenability of steel with higher alloy content or. Below temperature 230C the transformation appears to be independent of time and is only a function of temperature.
A curve is drawn joining the points for the times at which transformation begins and another for the times of finish of transformation as in Fig. For points below 230C the sub- critical bath is kept at that temperature. Time-Temperature-Transformation Curves TTT curves are a way of plotting transformation kinetics on a plot of temperature vs.
A point on a curve tells the extent of transformation in a sample that is transformed isothermally at that temperature. A TTT diagram usually shows curves that connect points of equal volume fraction transformed. 83205 L12 12706 9.
Fraction of transformation Vs the logarithm of time at constant temperature The S Curve. The transformation starts but it takes some time before we can see a precipitate. The time required for transformation required to initiate the transformation is known as Incubation Period.
TTT stands for time-temperature transformation which is basically a time-temperature transformation curve. CCT curves are useful to obtain different metastable products by controlling the rate of cooling. TTT is only for experimental purposes.
In the CCT the temperature is changing continuously we get phase transformation at continuous cooling. This is Lecture-17 on Material Science discussing very important Time-temperature-transformation curve TTT curve TTT diagram Part B which is very impo. Represents the time taken at any given temperature for a given fraction of the transformation to get completed.
The typical TTT diagram is a C shaped curve. In this case we have used it to represent at any given temperature the time required for 1 transformation to complete. At a temperature between 300 C and 550C the steel is in the form of bainite which is observed from a time temperature transformation diagram which shows the transformation time and temperature of transformation.
Get Time Temperature Transformation TTT Curve Multiple Choice Questions MCQ Quiz with answers and detailed solutions. Download these Free Time Temperature Transformation TTT Curve MCQ Quiz Pdf and prepare for your upcoming exams Like SSC Railway UPSC State PSC. Time temperature transformation T-T-T plots are also known as isothermal transformation diagrams.
The sweeping curve going through the centre of the graph opposite is the transformation curve which describes the extent of material which has changed through to pearlite. Timetemperaturetransformation TTT diagrams are proposed for the crystallization of amorphous metal oxide thin films and their specific characteristics are discussed in comparison to glass-based materials such as glass-ceramics and metallic glasses. The films crystallize from amorphous to full crystallinity in the solid state.